-
Ca2+ plays a pivotal role in cardiac physiological activities including contraction (Bers 2002; Szedlak et al. 2023). In cardiac myocytes, the sarcoplasmic reticulum (SR) serves primarily as the intracellular Ca2+ store providing most of the contractile Ca2+ for the myofilaments (Bers 2002; Lu et al. 2020). Ca2+ release from the cardiac SR is gated by type 2 ryanodine receptors (RyR2), the main SR Ca2+ release channels located on the membrane of junctional SR cisternae, which generates multiscale and multimodal cytosolic Ca2+ signals in the cardiac myocytes to mediate the cellular activities (Cheng and Lederer 2008; Xie et al. 2010). Dysfunctional gating of RyR2 due to stress modification or defective mutation of the channels will cause extra SR Ca2+ release in diastole that is termed as “SR Ca2+ leak”, which was first recognized in ventricular myocytes as an essential contributing factor to various ventricular pathological processes, such as heart failure and catecholaminergic polymorphic ventricular tachycardia (CPVT) (Huang et al. 2021; Marks 2023; Miotto et al. 2022; Mohamed et al. 2018; Steinberg et al. 2023). In recent years, more and more studies including ours have also revealed such SR Ca2+ leak in atrial myocytes promoting atrial fibrillation (AF) (Liu et al. 2020; Shan et al. 2012; Shoemaker et al. 2022; Tarifa et al. 2023; Zhang et al. 2021). With increasing interest in SR Ca2+ leak in atrial myocytes, to develop more accurate approach for atrial Ca2+ measurement is required due to obvious differences between atrial and ventricular Ca2+ handling (Bootman et al. 2011; Walden et al. 2009).
Since the discovery of the Ca2+ spark in 1993, the elementary intracellular Ca2+ event in cardiac myocytes has brought enormous information in deciphering the SR Ca2+ handling (Brochet et al. 2012; Cheng and Lederer 2008). In the past two decades, the spontaneous Ca2+ spark properties (frequency, total signal mass, etc.) were widely used as a readout to assess SR Ca2+ leak in ventricular as well as atrial myocytes (Huang et al. 2021; Lu et al. 2022; Shan et al. 2012; Zhang et al. 2021). However, Ca2+ sparks reflect only the components of SR Ca2+ release events with higher amplitude due to a cut-off for Ca2+ spark detection being introduced in order to exclude noise from signals (Brochet et al. 2011; Tomek et al. 2023; Yang et al. 2021). In fact, the total Ca2+ amount in subthreshold events is comparable to that of suprathreshold Ca2+ sparks (Brochet et al. 2011; Yang et al. 2021). Besides, compound Ca2+ sparks are also very common in atrial myocytes (Woo et al. 2003), which has also brought difficulty to the measurement of Ca2+ spark properties. An alternative measure of SR Ca2+ leak is to examine the effects of the RyR2 inhibitor tetracaine on diastolic Ca2+ levels in the absence of Na+ and Ca2+ in extracellular solutions (Mohamed et al. 2018; Zhang et al. 2021), which is, however, far from ideal due to the complex operation and non-physiological condition introduced.
In this work, we proposed two new parameters to quantify total SR Ca2+ leak in atrial myocytes using line-scan confocal Ca2+ images. These parameters showed well positive correlations with the classical readouts by Ca2+ sparks, while avoiding the complex process of detecting and analyzing Ca2+ sparks.
-
Wild-type (WT) and CPVT-mutated RyR2-R2474S+/− (R2474S) mice (Shan et al. 2012; Xie et al. 2013, 2015; Yang et al. 2021) were housed in a pathogen-free environment under a 12 h/12 h light-dark cycle and fed a rodent diet ad libitum. All strains of mice were on the same C57Bl/6 genetic background. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Xi’an Jiaotong University and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
-
Atrial myocytes were isolated from 3-month-old male WT and R2474S mice as previously described (Shan et al. 2012; Zhang et al. 2021). Briefly, after mouse was sacrificed, the heart is rapidly isolated, cannulated and subjected to Langendorff perfusion with perfusion buffer, comprised of (mmol/L): NaCl 113, KCl 4.7, KH2PO4 0.6, Na2HPO4 0.6, MgSO4 1.2, NaHCO3 12, KHCO3 10, HEPES 10, taurine 30, glucose 1.5 and 2,3-Butanedione monoxime 10, for 5 min at a speed of 3 mL/min. Then, the perfusate was switched to digestion buffer (includes 0.65 mg/mL collagenase type 2 and 50 µmol/L CaCl2 in perfusion buffer) and perfused for another 10–15 min. After enzymatic digestion of the heart is complete (heart appears swollen, pale and flaccid), cut down the atria and tease them into small pieces in stop buffer (includes 0.65 mg/mL collagenase type 2, 0.065 mg/mL protease XIV, 15 mg/mL BSA and 50 µmol/L CaCl2 in perfusion buffer) and bath at 37 °C for 10 min, then use pipets to dissociate the heart tissue gently until all the large pieces are dispersed. After being separated from the enzyme by centrifuging for 4 min at 10 g, cells are resuspended in Ca2+-free Tyrode solution (in mmol/L): NaCl 137, KCl 5.4, MgCl2 1.2, NaH2PO4 1.2, HEPES 20, glucose 10, titrated to pH 7.4 with NaOH. The [Ca2+] was then gradually recovered to 1.8 mmol/L and atrial myocytes were kept in the solution until the use.
-
Atrial myocytes are pre-loaded with 5 µmol/L fluo-4 AM for 15 min at room temperature, then washed out and kept in Tyrode solution containing 1.8 mmol/L CaCl2. A Leica TCS SP8 confocal microscopy with 63×/1.4 NA oil immersion objectives was used for confocal Ca2+ imaging. Cells were paced at 1 Hz lasting 1 min to normalize the store content. Line-scan images were acquired with the scanline along the long axis of cells at 400 lines/s for 20 s right after pacing. Scan zoom was fixed at 4.0 ( ~0.071 μm/pixel × 1024 pixels). The excitation for Fluo-4 is 488 nm, and emission is collected at 505–530 nm.
Ca2+ sparks were detected and analyzed using previously custom-developed software (Yang et al. 2021). The Ca2+ spark frequency and total signal mass, calculated by formula Σ((ΔF/F0) × FWHM3 × 1.206) (Brochet et al. 2011), are two classic readouts for SR Ca2+ leak, where ΔF/F0 represents the amplitude and FWHM (full width at half maximum) represents the spatial size of Ca2+ sparks.
-
As it has been shown that Ca2+ sparks reflect only the SR Ca2+ release event with amplitude above the cut-off threshold (Brochet et al. 2011; Yang et al. 2021), we developed two new parameters free of Ca2+ spark to quantify the SR Ca2+ leak from the confocal line-scan Ca2+ images.
The background noises of a confocal fluorescent image can be considered photon noise (von Wegner et al. 2006). After the line-scan Ca2+ image was normalized according to the row fluorescence without any processing for noises, a Gaussian distribution yields a good approximation to its background fluctuation (Bray et al. 2007; von Wegner et al. 2006). However, a slight rightward inclination is also notable in the background distribution (von Wegner et al. 2006). To better approximate the background fluctuations, we defined a modified Gaussian distribution:
where N0 represents the peak value of the histogram of normalized fluorescent intensity f distribution and σi denotes the fitting standard derivation (i = 1 for f < 1 and i = 2 for f ≥ 1 with σ1 is usually a little smaller than σ2). As shown in Fig. 1A, in the presence of 10 μmol/L tetracaine blocking Ca2+ leak via RyR2, the histogram of normalized fluo-4 fluorescence in confocal line-scan images can be well fitted into the modified Gaussian curves. After the removal of tetracaine, there was a notable difference between the raw fluorescence histogram curve and fitting curve in the lower right part corresponding to higher fluorescent intensity, which reflects the Ca2+ release signals (i.e., SR Ca2+ leak) in the line-scan images. We defined a new dimensionless parameter to quantify the difference between both curves as follows:
where H(f) denotes the raw histogram curve of normalized fluo-4 fluorescence, G(f) represents the fitting Gaussian curve with Eq. 1, and S equals to the area of the confocal line-scan Ca2+ image.
Notably, Fsignals reflects the summation of signal fluorescence, including both rising and declining phases of the signal pixels, which may bring errors due to overestimation of release signals. To exclude the declining phase of the signals, we calculated the temporal difference for the normalized line-scan Ca2+ images:
where Δt equals to the scan time for each line.
Then, pixels in lines-scan images with positive Δf (x, t) were counted up for histogram statistics and following Gaussian fitting. Thus, we defined another new dimensionless parameter corresponding to the summation of the rising phase of signal fluorescence as
where H’(f ) denotes the fluorescent histogram curve of pixels with positive Δf (x, t) and G’(f) represents the fitting Gaussian curve.
The new parameters Fsignals and Rsignals were tested for their effect in quantifying SR Ca2+ leak in atrial myocytes by comparing with Ca2+ spark frequency and total signal mass.
-
Data are presented as mean ± SD as well as individual points. Statistical analyses were carried out using Prism software version 9.0 (GraphPad Software Inc.). For continuous variables, statistical analysis was performed using a two-tailed unpaired student’s t-test. Differences were considered statistically significant at P < 0.05. All tests were 2-sided.
-
Intracellular Ca2+ plays a pivotal role in the cellular electricity and excitation-contraction coupling in atrial myocytes myocytes (Nattel et al. 2008). Abnormal Ca2+ handling in atrial myocytes has been closely associated with atrial diseases such as AF (Nattel et al. 2008). Similar to ventricular myocytes, the confocal fluorescent Ca2+ imaging has been the most commonly used approach for the measurement of intracellular Ca2+ signals in atrial myocytes. A typical confocal fluorescent image is formed by background noises and signals. To separate the signal pixels from the noisy ones in the line-scan Ca2+ images of atrial myocytes, we defined a modified Gaussian function to fit the histogram of fluorescent background fluctuations. With 10 μmol/L tetracaine blocking RyR2, the main Ca2+ channels in the SR of atrial myocytes, no signal but noises existed in the fluo-4 fluorescent images, in which the raw fluorescent histogram traces were well fitted into defined Gaussian curves, while Ca2+ signals as well as obvious differences between both traces occurred in the Ca2+ images in the absence of tetracaine (Fig. 1A). Correspondingly, the two new parameters calculated from the difference between raw histogram trace and fitting curve, Fsignals and Rsignals, were almost equal to 0 in case of complete inhibition of SR Ca2+ release signals with tetracaine and significantly increased in the absence of tetracaine (Fig. 1B). These data suggested that the present processing is effective to distinct Ca2+ signal components from background noises in the line-scan fluorescent Ca2+ images.
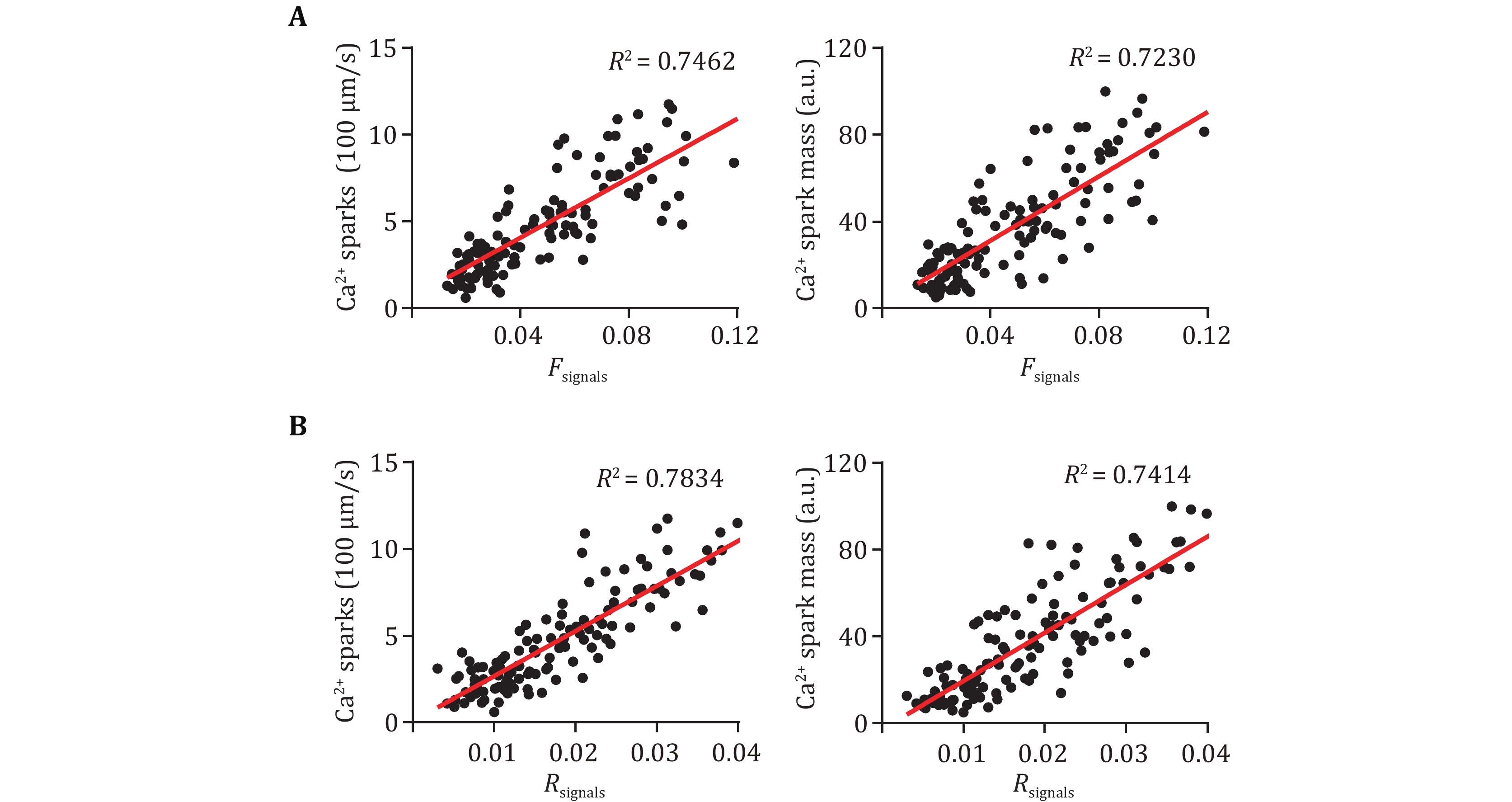
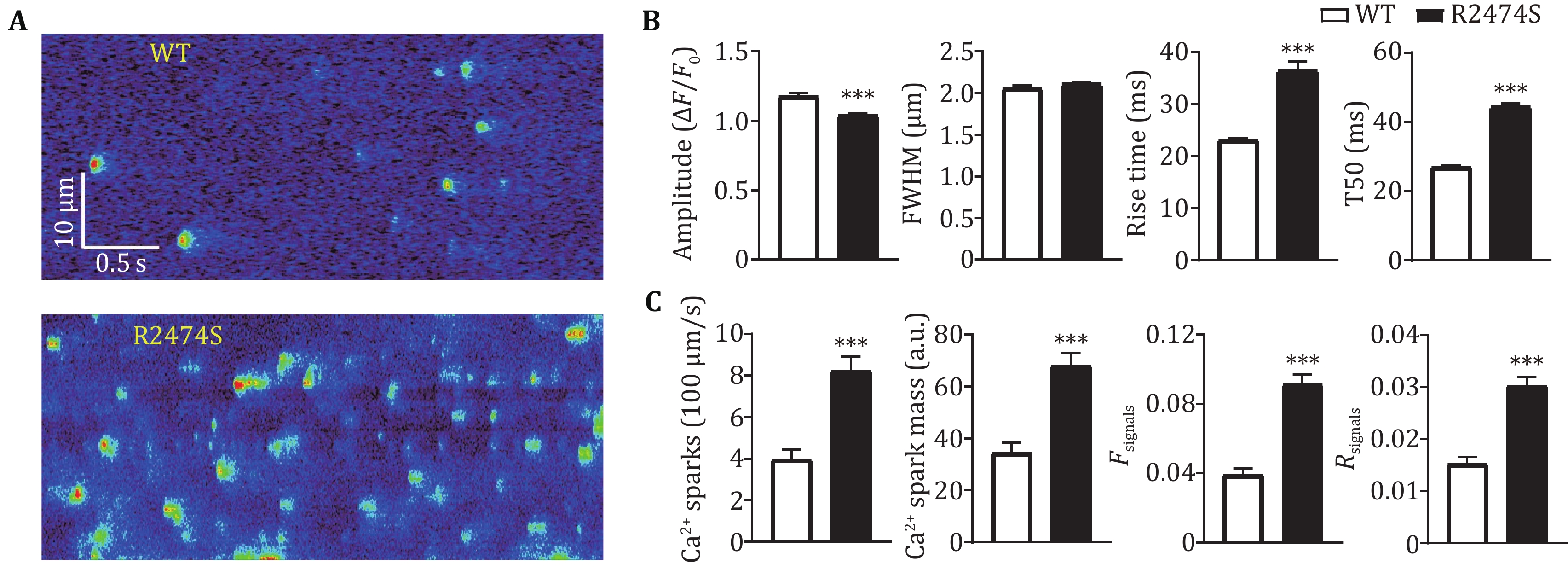
Accumulating evidence proved increased SR Ca2+ leak in ventricular as well as atrial myocytes essentially contributing to various cardiac diseases, such as CPVT and AF (Dridi et al. 2020; Houser 2023). Several approaches have been developed to quantify the SR Ca2+ leak in cardiac myocytes, among which Ca2+ spark characteristics are the most commonly used readouts. To further verify if the two new parameters can effectively reflect the SR Ca2+ leak in atrial myocytes, we performed the correlational analyses between them and Ca2+ spark frequency and total signal mass. More than 100 atrial myocytes from WT and R2474S mice were loaded with fluo-4 AM and conducted confocal line-scan Ca2+ imaging. As the Ca2+ release events are more active in atrial myocytes than ventricular myocytes (Shan et al. 2012; Walden et al. 2009), we use a recently developed threshold-free approach (Yang et al. 2021) to detect as many Ca2+ sparks, especially those with weaker amplitude. As shown in Fig. 2, both the two Ca2+ spark-free new parameters, Fsignals and Rsignals showed very good positive correlations with the Ca2+ spark frequency and total signal mass. R2474S mice harbored a well-known “leaky” RyR2, which displayed both increased AF susceptibility and SR Ca2+ leak in atrial myocytes (Shan et al. 2012). Consistent with previous results, the Ca2+ spark frequency and total signal mass in atrial myocytes from R2474S mice are 1.94 and 2.05 fold of that in WT groups (Figs. 3A–3C). A similar conclusion was achieved by using the new parameters, Fsignals and Rsignals, which are 2.41 and 1.96 folds in the R247S group than in the WT group. These results proved that our newly developed two parameters are effective in quantifying SR Ca2+ leak in atrial myocytes and can be used as readouts to distinguish atrial myocytes under normal and pathological conditions.
As the elementary SR Ca2+ release event in cardiomyocytes, Ca2+ spark has been commonly used as readouts to quantify SR Ca2+ release (Brochet et al. 2011; Shan et al. 2012; Zhang et al. 2021). However, the spontaneous Ca2+ sparks are usually more frequent in atrial myocytes than in ventricular myocytes (Shan et al. 2012). For example, in atrial myocytes from R2474S mice, there exist plenty of compound Ca2+ sparks as well as lower-amplitude Ca2+ sparks (Fig. 3A), which bring out great difficulties for precise detection and characterization of these events. Besides, the temporal characteristics, including rise and decay time, of Ca2+ sparks are obviously greater in the R2474S group compared to the WT group (Fig. 3B), which reflects a prolonged release or multiple release even in the decay phase in R2474S atrial myocytes (Brochet et al. 2011). Obviously, these post-peak leaky components were ignored with Ca2+ spark readouts. Avoiding the trouble for Ca2+ spark detection and analyses in atrial myocytes, the two new parameters can be easily calculated. Besides, while the change rate (1.96) of Rsignals is almost the same as Ca2+ spark frequency and total signal mass between the WT and R2474S group, the change rate (2.41) of Fsignals is a little greater, which suggests that the parameter can include the prolonged SR leak components (Fig. 3C). An alternative approach for the measurement SR Ca2+ leak is the protocol developed by Shannon et al. (Zhang et al. 2021). However, since Ca2+ leak is a spontaneous opening event of a small number of RyR2, which occupy only a small part (about 5%) of the total SR Ca2+ load, such a huge difference between SR Ca2+ leak and load can also lead to inaccuracies for the fluorescent measurements. Moreover, the complex operation and non-physiological conditions introduced have also made it far from ideal.
Although our algorithm needs to fit the background noise, it’s not limited to processing images with special signal-to-noise ratios (SNR), in fact, the line-scan Ca2+ images in Figs. 2–3 have been acquired at the confocal microscope with SNR ranging from ~1.6 to ~3.4. While we have focused on atrial myocytes, our algorithm can also be used to detect the SR Ca2+ leak in other tissues, such as ventricular myocytes or skeletal muscle cells, since there is no special processing for atrial myocytes in this approach. Ca2+ wave is another type of SR Ca2+ release, formed by Ca2+ sparks propagating along cells. Whether our algorithm is suitable to quantify SR Ca2+ leak via Ca2+ waves calls for further assessment.
Taken together, the present study proposed a simple and effective approach for quantifying SR Ca2+ leak in atrial myocytes, to facilitate research on calcium signaling in atrial physiology and diseases.
Assaying sarcoplasmic reticulum Ca2+-leak in mouse atrial myocytes
- Received Date: 06/12/2023
- Available Online: 31/10/2024
-
Key words:
- Atrial myocyte /
- Sarcoplasmic reticulum /
- Ca2+ leak /
- Confocal images /
- Ca2+ measurement
Abstract: More and more studies have suggested an essential role of sarcoplasmic reticulum (SR) Ca2+ leak of atrial myocytes in atrial diseases such as atrial fibrillation (AF). The increasing interest in atrial Ca2+ signaling makes it necessary to develop a more accurate approach for Ca2+ measurement in atrial myocytes due to obvious differences between atrial and ventricular Ca2+ handling. In the present study, we proposed a new approach for quantifying total SR Ca2+ leak in atrial myocytes with confocal line-scan Ca2+ images. With a very precious approximation of the histogram of normalized line-scan Ca2+ images by using a modified Gaussian distribution, we separated the signal pixel components from noisy pixels and extracted two new dimensionless parameters, Fsignals and Rsignals, to reflect the summation of signal pixels and their release components, respectively. In the presence of tetracaine blocking SR Ca2+ leak, the two parameters were very close to 0, and in atrial myocytes under normal conditions, the two parameters are well positive correlative with Ca2+ spark frequency and total signal mass, the two classic readouts for SR Ca2+ leak. Consistent with Ca2+ Spark readouts, the two parameters quantified a significant increase of SR Ca2+ leak in atrial myocytes from mice harboring a leaky type 2 ryanodine receptor mutation (RyR2-R2474S+/−) compared to the WT group. Collectively, this study proposed a simple and effective approach to quantify SR Ca2+ leak in atrial myocytes, which may benefit research on calcium signaling in atrial physiology and diseases.

 首页
首页 登录
登录 注册
注册



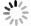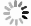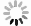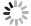
 DownLoad:
DownLoad: